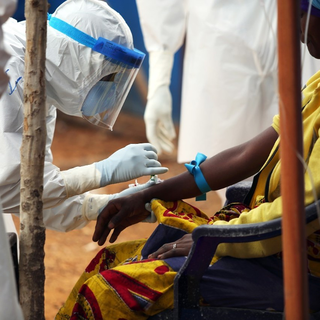
By Kali Kotoski, Midwest AViDD Center Communications Director
Introduction
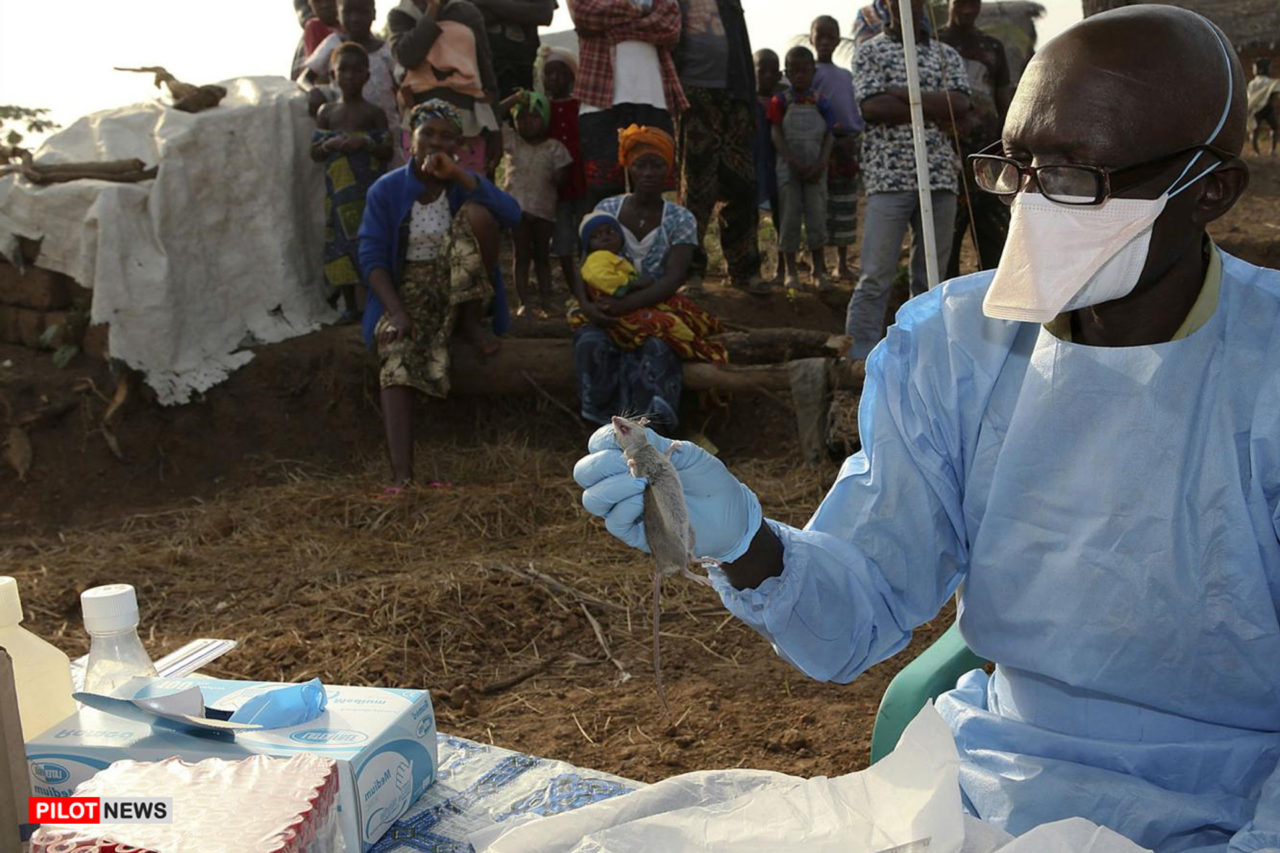
Lassa fever is an animal-borne arenavirus that causes acute viral illness. It is spread by the common African rat and is endemic in parts of West Africa, including Benin, Ghana, Guinea, Liberia, Mali, Nigeria and Sierra Leone, and other West African countries. There have also been cases seen in the USA and Europe imported from West Africa.
The first documented case occurred in 1969 in Lassa, Nigeria, hence its name.
Conservatively, 100,000 to 300,000 infections of Lassa fever occur annually, resulting in about 5,000 deaths. Surveillance for Lassa fever is sporadic, making estimates crude. In some areas of Sierra Leone and Liberia, about 10 to 16 percent of people admitted to hospitals annually have Lassa fever. The case fatality rate is believed to be between 1 and 15 percent. However, in hospitalized cases, the fatality rate ranges from an alarming 21 to 69 percent.
Transmission
Garry, R.F. Lassa fever — the road ahead. Nat Rev Microbiol 21, 87–96 (2023). https://doi.org/10.1038/s41579-022-00789-8
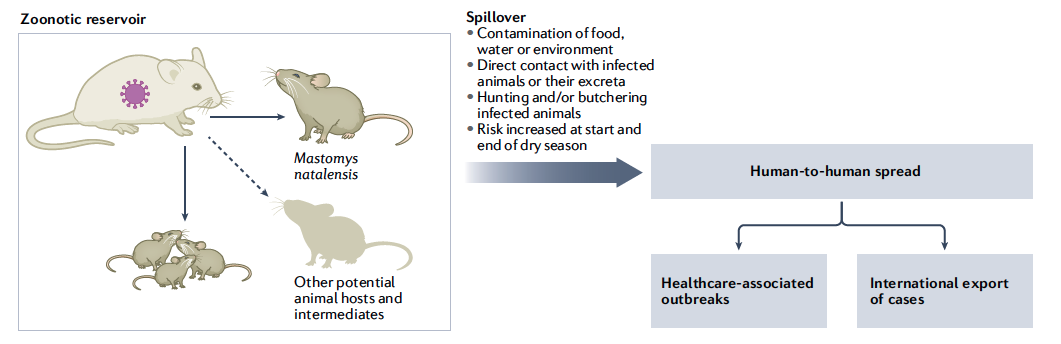
The host of the Lassa virus is a rodent from the multimammate rodent species called either Natal multimammate mouse, Natal multimammate rat, or African soft-furred mouse. Once infected, this rodent can excrete the virus in urine for perhaps the rest of its life. Breeding frequently and producing copious offspring, the rodent’s natural habitat comprises savannas and forests of West, Central, and East Africa. Despite the multimammate rodent species habitat throughout sub-Saharan Africa, Lassa fever has not been found in rodents outside of West Africa, perhaps due to historical bottlenecks in the dispersal of the virus, reservoir, or both, according to the 2014 edition of Emerging Infectious Diseases.
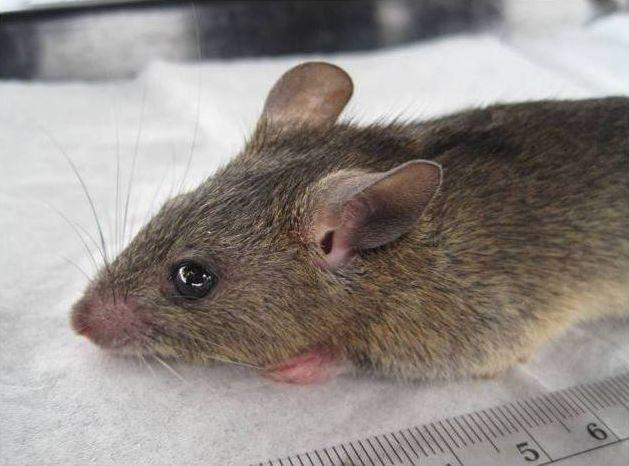
Transmission of Lassa virus to humans occurs most commonly through ingestion or inhalation of rat urine or droppings. Exposure to open cuts or sores can lead to infection.
The rat readily colonizes human homes and contaminates areas where food is stored by salivating or urinating on rice, cassava, and other crops stored in barrels or left to dry in the sun. The virus can also be transmitted by eating an infected rat that has not been thoroughly cooked.
“The rat is actually a delicacy,” explained Christian Happi, director of the African Center of Excellence for Genomics of Infectious Diseases in Osun State, Nigeria, in a 2018 interview with Vox. “People in remote areas of Africa will eat [the rats] without a second thought. It’s a major source of protein.”
Contact with the virus may also occur when a person inhales tiny particles in the air contaminated with infected rodent excretions, according to the Centers for Disease Control and Prevention (CDC). This aerosol or airborne transmission may occur during cleaning, such as sweeping.
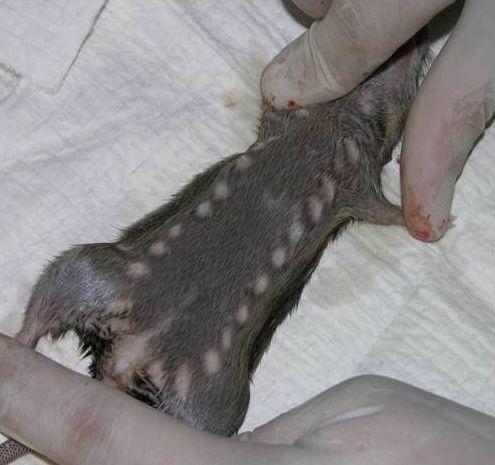
Person-to-person transmission is common in health care settings (called nosocomial transmission) after exposure to the virus in a Lassa virus-infected individual's blood, tissue, secretions, or excretions.
History of Outbreaks
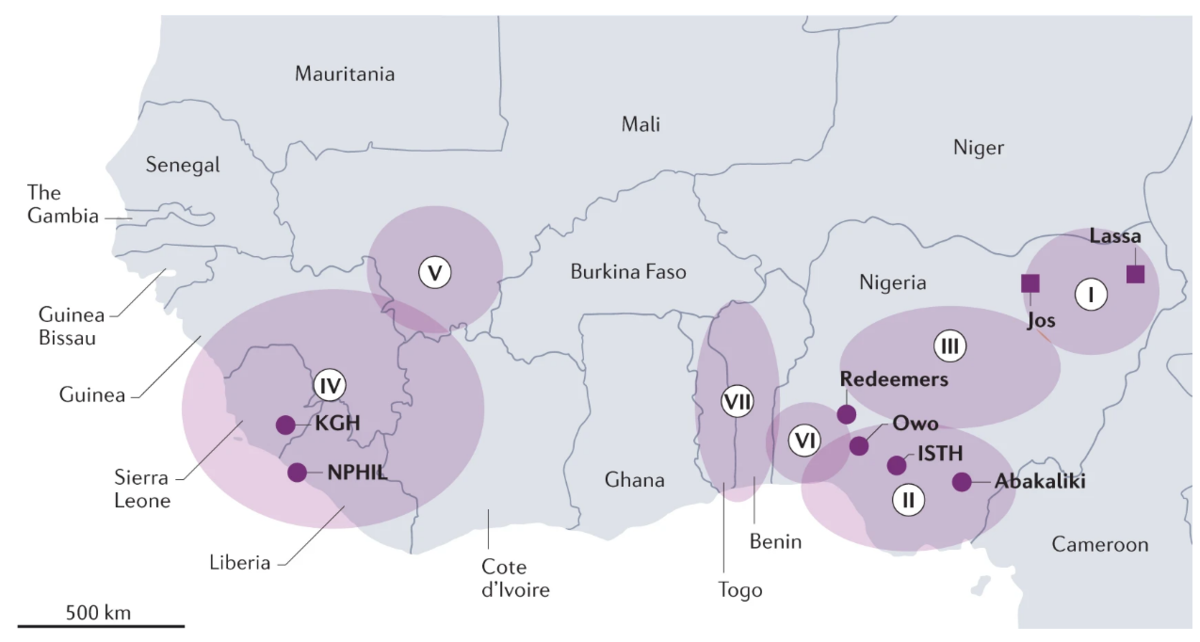
Garry, R.F. Lassa fever — the road ahead. Nat Rev Microbiol 21, 87–96 (2023). https://doi.org/10.1038/s41579-022-00789-8
Genetic dating suggests that the Lassa virus could have been infecting humans for centuries. In the 1930s, French colonizers dubbed a potential Lassa fever infection "savanna typhus” because it occurred in the dry season, was prevalent in people hunting small game, like rodents, and had a high mortality rate of 50 percent. However, it was not until 1969 that the first patient with Lassa fever was recognized—a missionary nurse working in Lassa, Nigeria, who died on the way to a hospital in the city of Jos. There, two other nurses contracted the virus and died, while a third nurse was taken to New York City to have her blood examined at Yale University, thus isolating Lassa fever. The nurse survived, but two Yale researchers got sick, one fatally.
It wasn’t until an investigation of an outbreak in the early 1970s in Sierra Leone determined that the multimammate rodent species was the major carrier of the virus, which is transmitted to offspring.
Although animal models for Lassa fever have been around since the 1980s, the decade-long civil war in Sierra Leone (from 1991 to 2002) hampered on-the-ground research.
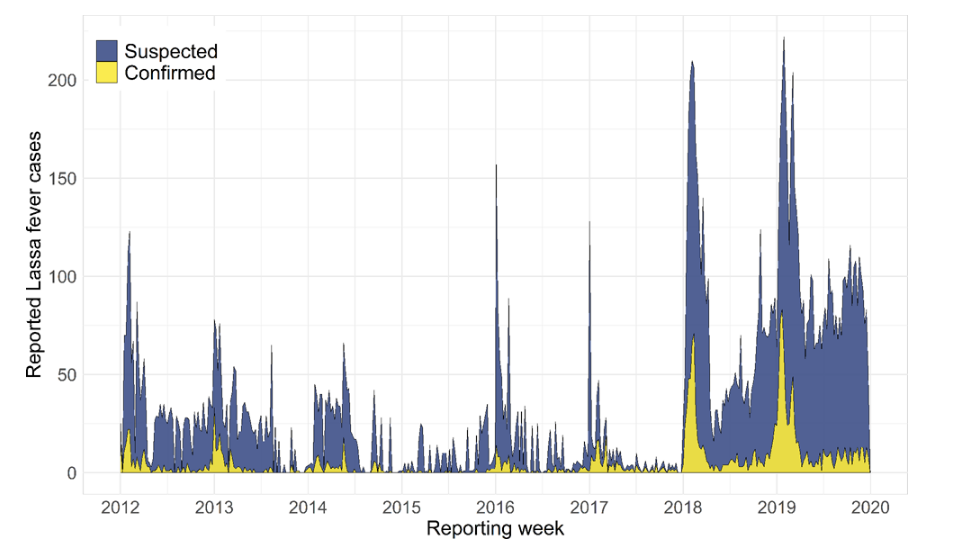
Temporal trends in country-wide Lassa fever case reporting from 2012 to 2019 in Nigeria. Redding, D.W., Gibb, R., Dan-Nwafor, C.C. et al. Geographical drivers and climate-linked dynamics of Lassa fever in Nigeria. Nat Commun 12, 5759 (2021). https://doi.org/10.1038/s41467-021-25910-y
From 2018 until now, Nigeria and neighboring countries have seen annual endemic outbreaks. As of May 1, 2023, a Lassa outbreak in Nigeria has infected a suspected 4,702 people with 877 confirmed cases and 152 deaths, representing a 20 percent increase in confirmed cases compared to the same period in 2022.
"It can be emotional seeing a patient who you could have helped if the person had come earlier, but as long as you are doing everything possible to save the patient, it somehow takes the emotion off you," Dr. Fyne Akubueze, a frontline healthcare worker southwest Nigeria, told Gavi. Gavi is a vaccine alliance that partners with governments and is funded by the Bill and Melinda Gates Foundation, UNICEF, WHO, and The World Bank.
Symptoms

Symptoms of Lassa fever are typically noticeable after one to three weeks after infection. For most Lassa fever infections, symptoms are mild, undiagnosed, and similar to other febrile illnesses. The case fatality rate is believed to be between 1 and 15 percent. However, in hospitalized cases, the fatality rate ranges from an alarming 21 to 69 percent.
Mild symptoms include slight fever, general malaise and weakness, and headache. The disease may progress to more severe symptoms, including hemorrhaging (bleeding from the gums, eyes, or nose), respiratory distress, repeated vomiting, facial swelling, pain in the chest, back, and abdomen, and shock. Neurological problems include hearing loss, tremors, and encephalitis. Death may occur within two weeks due to multi-organ failure.
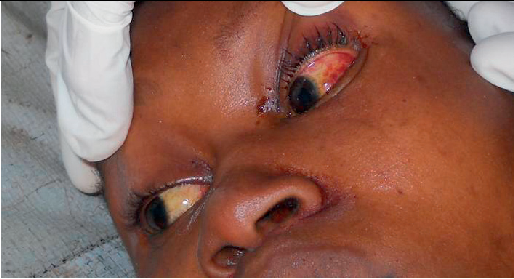
The most common complication of Lassa fever is deafness, occurring in one-third of infections and often permanent. Deafness may develop in mild as well as severe cases.
The death rates for pregnant women are three times greater, and during early pregnancy, 92 percent of fetuses are spontaneously aborted from infected mothers, and 72 percent are aborted in the third trimester. The reason is that the Lassa virus thrives in the placenta, fetal tissues, and maternal blood at higher viral loads. One of the reasons researchers believe it is so deadly to pregnant mothers and their fetuses is because of misdiagnosis and hampered immunity. In late-trimester patients, symptoms can mimic eclampsia, obstetric hemorrhagic, and pregnancy-related sepsis. A Lassa infection also is often mistaken for malaria, given the similar symptoms. Given the vulnerability of pregnant women, safe antivirals and vaccines must be created to protect against or neutralize an infection during pregnancy.
What is the Structural Biology of the Lassa Virus?
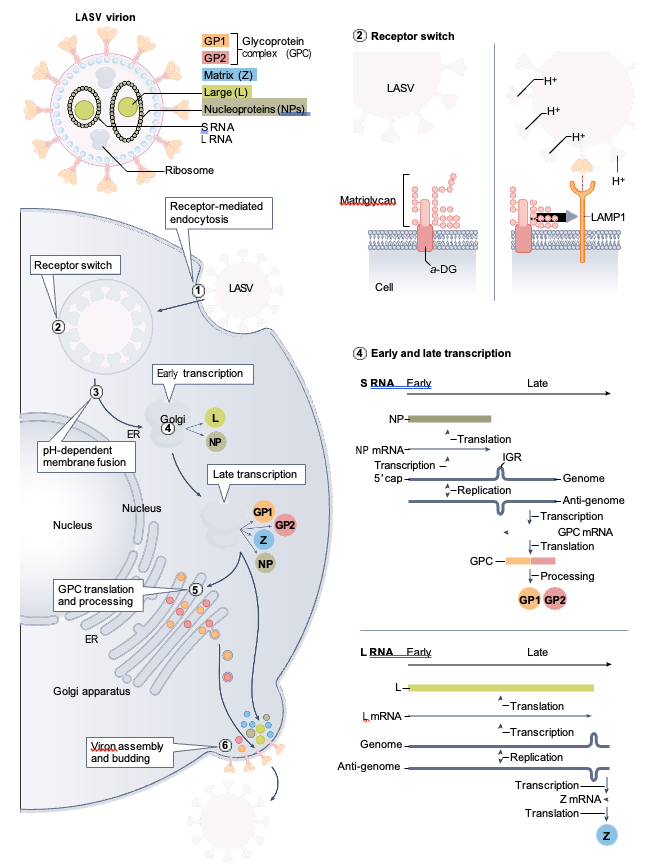
Garry, R.F. Lassa fever — the road ahead. Nat Rev Microbiol 21, 87–96 (2023). https://doi.org/10.1038/s41579-022-00789-8
Lassa virus is often described as a small minimalist virus with two single-stranded RNA segments that encode two sets of proteins. When looked at, the virus appears to be filled with sand, which is actually ribosomes. The role these ribosomes play in the virus's infection and replication cycles remains a mystery.
The four proteins serve different functions. The nucleoprotein encapsulates the viral genome and is essential for both transcription and replication of the virus. The glycoprotein works on viral attachment to a cell and cell entry. The Large protein is an RNA polymerase involved in transcription and replication. The Zinc- binding (Z) protein helps with viral assembly. Once the virus binds to a cell, it is internalized through endocytosis.
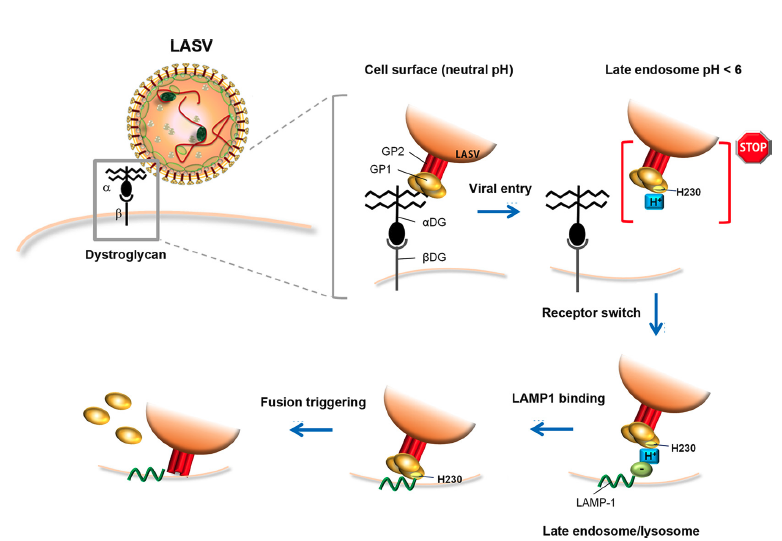
At the cell surface, LASV GP1 engages the O-linked matriglycan polysaccharides displayed by α-DG, followed by endocytosis via an unusual pathway related to macropinocytosis. Progressive acidification of late endosomes induces a structural change in LASV GP1, which dissociates from DG and adapts a low-pH conformation displaying a histidine triad. Protonation of residue H230 “locks” GP1 in the prefusion state, preventing premature fusion. Engagement of LAMP1 neutralizes the positive charge on H230 of GP1 and triggers efficient fusion with the limiting membrane of the late endosome/lysosome. Lassa Virus Cell Entry Reveals New Aspects of Virus-Host Cell Interaction Giulia Torriani, Clara Galan-Navarro, and Stefan Kunz
The first viral proteins to be made in the cell are the Large (L) protein and the nucleoprotein, which are sufficient to synthesize antigenomes. The antigenomes serve as a template for RNA encoding. Once the virus has finished replicating, it is released from the cell membrane and looks for another cell to infect.
The Lassa virus has undergone several mutations and continues to spread and diversify. Currently, there are seven known viral lineages with mutations concentrated in protein epitopes. The mutations show an increase in transmissibility among multimammate rodent populations from enhanced viral escape.
Why is the Lassa Virus Categorized as a Virus of Pandemic Concern?
Lassa virus has been recently ranked as posing the highest threat of a spillover event because of the abundance of the multimammate rodent species across Africa. While human-to-human transmission is possible by exchanging bodily fluids, every virus risks mutating and becoming more infectious or a human contagion.
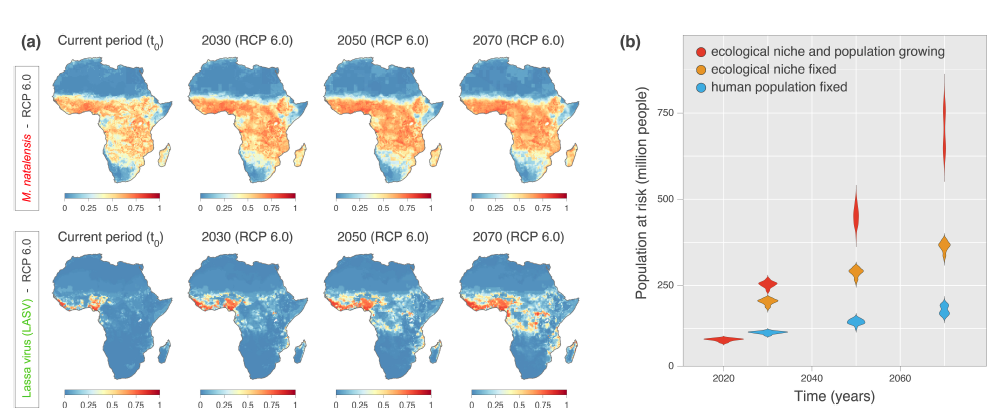
Klitting, R., Kafetzopoulou, L.E., Thiery, W. et al. Predicting the evolution of the Lassa virus endemic area and population at risk over the next decades. Nat Commun 13, 5596 (2022). https://doi.org/10.1038/s41467-022-33112-3
Regardless of whether a mutation like that will ever happen, scientists in a 2022 publication by Scripps Research and the University of Brussels revealed that temperature, rainfall, and pastureland are the leading contributors to viral transmission. By analyzing decades of environmental data, the researchers suspect that over the next 50 years, the virus could spread from West Africa into Central Africa and East Africa. With this expansion and population growth, people living in endemic areas could rise by more than 600 million. It is believed that between 39 million and 59 million people are currently at risk of Lassa virus infections across 14 countries. Other research models show that those living in a Lassa fever endemic area will be as high as 700 million by 2070.
However, many factors could make that outlook not possible. Because of Lassa's viral host, the virus has remained remarkably confined to West Africa. Despite genetic testing showing that the virus has likely been around for over a thousand years, it struggles to cross waterways, with different lineages from one side of a river to the other. That is the case in parts of Nigeria. While the multimammate rodent species is abundant across sub-Saharan Africa, mice in Central and Eastern Africa are not Lassa carriers. This puzzling situation has been theoretically explained as the virus has to compete with other viruses, which stops Lassa from getting a foothold in mice, or the mice have acquired Lassa antibodies. Another theory is that it only infects one (Natal multimammate mouse) of the six subspecies of the multimammate rodent species or that environmental determinants have a massive impact and can limit the abundance of the host, which can drive the population of the host to below the threshold needed for viral maintenance. The Natal multimammate mouse is believed to have a cellular immune response that protects it from the Lassa virus infection. In contrast, studies in other rodents and non-human primates show that an infection can be fatal.
Nevertheless, researchers believe that the virus could spread to include a much larger populous as climate change alters precipitation levels, as that is the most significant factor in the population of the multimammate rodent species. Because Lassa fever thrives at the beginning of the dry season and the Natal multimammate rat breeds in the wet season, less frequent rainfall, yet more intense, could aid the virus’s spread. The Natal multimammate rat is most active after the rains have ended and food sources are bountiful.
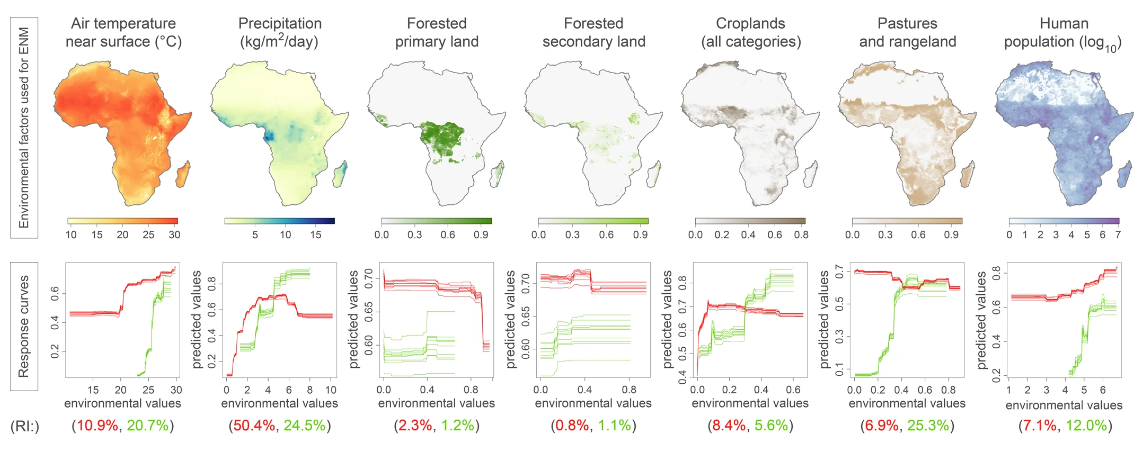
Response curves and relative importance (RI) obtained for the ENM analyses of M. natalensis and Lassa virus are coloured in red and green, respectively. The ten response curves reported for each ENM analysis correspond to ten independent repetitions of the boosted regression trees (BRT) analysis. These response curves indicate the relationship between the environmental values and the response (i.e., the ecological suitability of M. natalensis or Lassa virus). In addition to the seven environmental factors displayed in this figure, two additional factors were also included in the ENM analyses, the non-forested primary land, and non-forested secondary land.
Another growing concern is that the virus has been isolated in at least three pigmy mice in Benin and Togo, leading researchers to wonder if the virus could have a new viral host. The Lassa virus has also been found in lower concentrations in eight other multimammate rodent species. It also has been shown to infect the black rat, bush rat, house mouse, and shrews during experiments.
Outlook on Treatments
Although there are no approved drugs or vaccines for Lassa fever, the antiviral ribavirin has been promoted as the best current clinical approach. Ribavirin was designed to treat Hepatitis C. Its effectiveness as post-exposure prophylaxis has not been calculated for Lassa, as the rate of secondary transmission of infection after exposure is unknown. Nevertheless, it has been the clinical course of action for years and appears most effective when given early and intravenously. A newer drug, favipiravir — an oral viral RNA polymerase inhibitor licensed for influenza treatment in Japan — had shown promise in two individuals treated in a biocontainment unit when it was combined with ribavirin. Favipiravir has demonstrated success in treating infections of Lassa in guinea pigs, mice, and macaques.
However, like ribavirin, favipiravir should be avoided, if possible, in pregnant and breastfeeding women. The current standard treatment protocol for pregnant women—regardless of trimester—involves the intravenous administration of ribavirin. If the pregnant woman has a viable fetus, a conservative amount of ribavirin, along with antibiotics and a closely monitored delivery in a dedicated biocontainment unit is the best course of action. Post-delivery, the placenta and anatomical waste need to be carefully disposed of because they are highly contagious. If the newborn is asymptomatic of Lassa fever, ribavirin should not be used until further tests are completed. The use of baby formula instead of breastmilk is advised, depending on PCR tests of breastmilk.
Most recently, scientists have identified three major monoclonal antibodies — artificial proteins that act like antibodies in the immune system — that have successfully suppressed the virus even when administered as long as eight days after infection in embryonic human kidney cells and macaques.
The treatment, called Arevirumab-3, causes each of the three antibodies to bind to distinct regions of the Lassa Virus glycoprotein, which allows the virus to attach and enter the cell. Thus, by binding to the glycoprotein, the virus loses the ability to enter the cell. Researchers said this therapy, developed by La Jolla Institute for Immunology, could create a pathway for vaccine development.
Concerns over cost are at play when considering if the monoclonal antibody treatment could be a stand-alone therapy, as the existing price tag for such medicines is $200 to $250 a gram.
Another approach to treating Lassa fever infections is to find ways to boost T cell responses, as T cells play a more significant role in clearing an infection than antibodies. Researchers have concluded that antibody responses alone do not provide adequate protection. However, boosting T-cell responses could be accomplished through a vaccine or an antiviral.
As of 2022, there are nearly two dozen vaccine candidates in pre-clinical trials, with a few in Phase 1 human trials. There are also some promising antivirals being explored.
Midwest AViDD Center Work
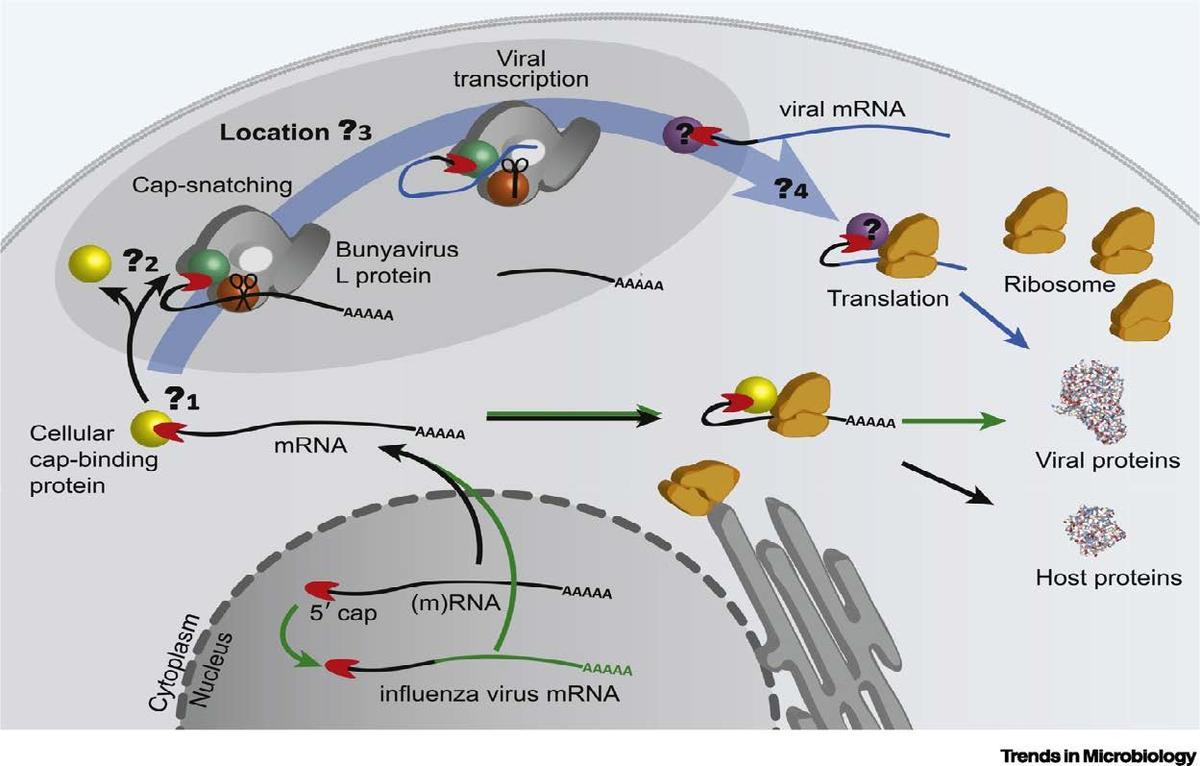
The “cap snatching” mechanism in LASV replication.
The Lassa virus is one of the viruses at the core of the research at the Midwest AViDD Center. At the center, two projects are developing small molecule viral entry inhibitors that, if successful, could yield a treatment that can be given post-infection and stops the replication of the virus, thus allowing the immune system to ramp up.
These projects are led by Michael Farzan and Hyeryun Choe of Harvard Medical School and Lijun Rong at the University of Illinois Chicago.
Midwest AViDD co-director Fang Li at the University of Minnesota and Lanying Du at Georgia State University are developing nanobody therapies designed to prevent the virus from infecting cells.
The University of Minnesota’s Hinh Ly, Hideki Aihara, Yuying Liang, and Iowa State's Yang Yang are developing antivirals from protease inhibitors that stop viral replication, as well as through nuclease inhibitors that could launch a new class of antiviral therapeutics.
Two mentored projects are also looking at creating antivirals by targeting the nucleoprotein function and the endonuclease of the Lassa virus.
This page will be updated with the Midwest AViDD Center's work as discoveries are made and papers are published.