
What are viruses of pandemic-level concern?
Explaining potential pandemic viruses and the Midwest AViDD Center’s work
By Kali Kotoski, Midwest AViDD Center Communications Director
Just over three years ago, nobody knew that SARS-CoV-2 even existed, let alone that 30,000 nucleotides of a single-stranded RNA virus would kill an estimated 20 million, bring the world’s economy to its knees, reorder everyday life, and disrupt modern society so profoundly that its scars are still being uncovered.
For researchers, the next pandemic was never a question of "if" but "when,” especially as there are a myriad of closely monitored and highly dangerous viruses a spillover or mutation away from an outbreak that could potentially ravage the globe.
While life-threatening coronaviruses are not new, with outbreaks of severe acute respiratory syndrome coronavirus (SARS-CoV) in China from late 2002 to late 2003 killing 700, and the Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia in 2012 killing 800, the devastation of the Covid-19 pandemic made viral pathogens a kitchen table issue.
Nobody was ready for the Covid-19 pandemic, but we were not entirely caught flat-footed because of the close monitoring of potential pandemic viruses (PPVs). The rapid scientific progress was inspiring for the public—vaccines in less than a year, monoclonal antibody treatments—but that was only because of decades of research that laid the groundwork to develop treatments for the novel virus quickly.
But coronaviruses are hardly the only viruses that researchers are concerned about.
When classifying PPVs, there are a host of characteristics that researchers look for. One, now part of the global lexicon, is an RNA virus genome instead of a DNA genome. RNA viruses typically don't have proofreading abilities—SARS-CoV-2 being an exceptional exception—and replication is less stable than DNA. This increases RNA viruses' mutation rates, making them more adaptable to human infection and transmission. However, DNA viruses can be profoundly devasting with smallpox as an example.
Researchers also look for dangerous pathogens with a track record of jumping from animals to humans. For example, Ebola and HIV have a zoonotic origin, and a study published by the United Nations Environment Programme and the International Livestock Research Institute in 2020 shows that about 60 percent of known infectious diseases in humans and 75 percent of all emerging infectious diseases are zoonotic.
A zoonotic leap, however, does not guarantee an epidemic or pandemic. The virus must then become transmissible from human to human, a process that increases in likelihood the more leaps a virus makes.
Traditionally, a zoonotic leap, or spillover, was believed to be like a virus winning the lottery. Still, a growing body of evidence claims that it is far more common, but the virus doesn't achieve human-to-human transmission, does not show apparent symptoms, or needs to be studied.
This leads to the third troubling factor: the mode of transmission. The most concerning is when a virus can spread through respiratory droplets that cause infection from close interactions. However, some of the deadliest potential pandemic viruses currently spread through sexual contact, blood, mucus, and feces.
The World Health Organization lists the most concerning viruses. At the same time, the Center for Disease Control includes influenza, such as avian flu, of great concern. Unfortunately, the WHO doesn't rank these viruses with a probability score, so the following list is not in order of the likelihood of an outbreak.
Influenza

The current bird flu outbreak – the biggest one ever recorded – has caused a massive number of avian deaths across the globe, is starting to infect mammals, and there are reported cases of human infection.
Bird flu has long been on infectious disease experts' radars since it was first identified in 1997 in Southern China and Hong Kong. Plus, some researchers believe the 1918 Spanish Flu – one of the worst pandemics in recent history – could have been caused by a strain of bird flu.
Since the 1918 flu, three notable flu pandemics have occurred from 1956 to 1957, 1968 and 2009. With this track record, it was widely suspected that the next pandemic would be caused by influenza.
So far, 15 million domestic birds have died from the flu over the past year and a half, and more than 193 million have been culled to stop H5N1 from spreading. Here in Minnesota, the avian flu outbreak has had a devastating effect on our native raptor community including bald eagles and owls, according to the University of Minnesota’s Raptor Center. More broadly, the disease has been found to infect bears, dolphins, foxes, mink and seals, making researchers concerned that it is mutating to spread amongst mammals. However, others believe it is likely that these animals contracted the virus from scavenging activity. Human infections have already been identified in parts of Asia but remain scarce and are linked to workers in the poultry industry.
Yet, according to the WHO, from 2003 until February of this year, a total of 240 cases of human infection have been reported from four countries within the Western Pacific Region since January 2003. Of these cases, 135 were fatal, resulting in a harrowing case fatality rate of 56 percent. But it is good to remember those infections were over two decades and mild cases of the disease are less likely to be diagnosed and reported.
While a vaccine exists for H5N1 for birds and humans, similar concerns around Covid-19 vaccines are being replayed, especially if it infects humans frequently—such concerns as mass production, stockpiles, distribution and hesitancy. Luckily, antivirals already work against the flu virus regardless of the strain. Still, they need to be administered early, which could make testing a top priority.
For researchers, it's clear that preparedness is critical and that there should be more investment in disease surveillance in poultry and pig farms. Others have argued that outbreaks must be contained and stamped out as quickly as possible and that poultry and pig farm workers should get vaccinated.
The current bird flu outbreak has a fatality rate of 90 to 100 percent among birds.
Covid-19 (SARS-CoV-2)

The current pandemic is believed to have a fatality rate of 1 to 2 percent. However, this is a data-driven estimate with many limitations. Not all cases have been reported, nor have all deaths, and the fatality rate has varied over the last three years as different strains emerged coupled with the development of vaccines and therapeutics. For example, according to the WHO, data suggested the original strain had an over 20 percent fatality rate when the virus was first discovered in Wuhan, China. But over the next few months, that number dropped precipitously.
The Midwest AViDD Center was created in the wake of the pandemic, and developing antivirals for Covid-19 is at the forefront of its research. From small molecule inhibitors (drugs) to nanobodies, protease inhibitors, helicase inhibitors, and nuclease inhibitors, the Midwest AViDD Center is exploring and developing drugs to treat this disease.
Crimean-Congo Hemorrhagic Fever
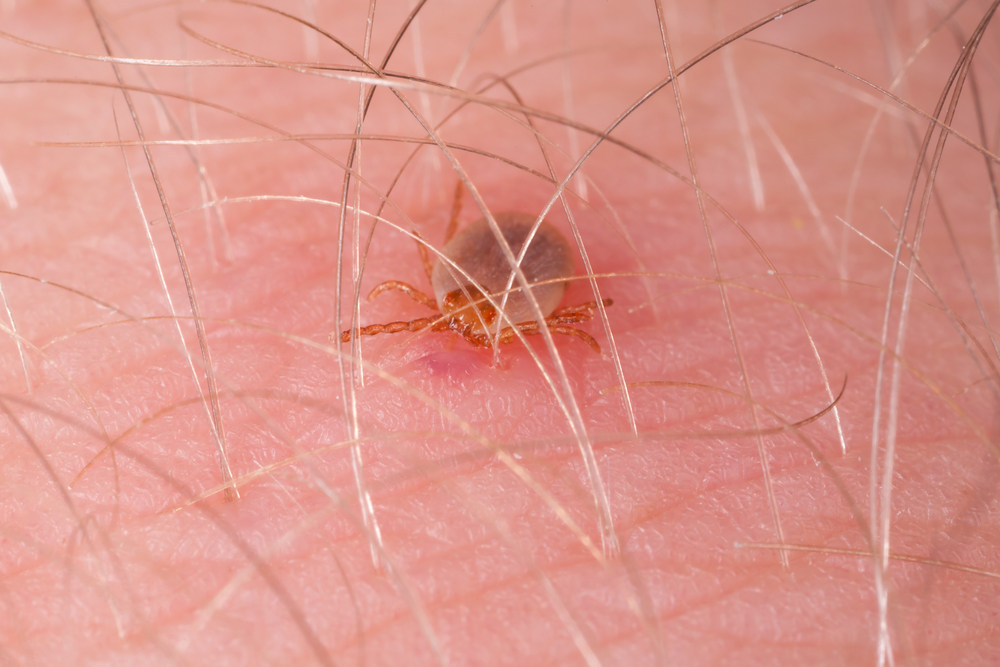
Possibly a prehistoric virus with archeological blood samples showing its existence in Germany as far back as 1500 BC, the Crimean-Congo Hemorrhagic Fever virus was first discovered in 1944 in Crimea. Still, it wasn't isolated until 1956 in Congo. Despite its name, it is endemic in Africa, the Balkans, the Middle East, and Asia, with a 10 to 40 percent fatality rate. Spread by ticks, infected livestock, and close contact with the blood or fluids of an infected person, the virus is believed to have evolved along with changing agricultural processes.
It causes severe outbreaks of viral hemorrhagic fever. This condition can damage the body's organs and cardiovascular systems, often including severe bleeding. The antiviral Ribavirin, created in 1972, is typically used to help treat infections while no vaccine currently exists.
Ebola Virus Disease and Marburg Virus Disease

Marburg and Ebola viruses are both filoviruses yet are distinct from each other. They cause clinically similar diseases characterized by hemorrhagic fevers and capillary bleeding. Because the viruses typically reside in non-human hosts, they fall into a category called zoonoses.
Ebola is found in primates such as monkeys, gorillas, chimpanzees, forest antelope, porcupines, and bats, with outbreaks occurring when handling a sick or dead animal in the rainforest. First appearing in Nzara, South Sudan, and near the Ebola River in Yambuku, Democratic Republic of Congo, two simultaneous outbreaks in 1976 put this extremely deadly virus on the map. It is believed that fruit bats may be Ebola’s primary host.
With case fatality rates varying from 25 percent to 90 percent depending on the strain and averaging 50 percent, Ebola spreads through human-to-human transmission through blood and body fluids from broken skin or mucous membranes. To top it off, it can also be spread from handling dead bodies of Ebola victims, making funeral practices a significant concern for community spread. In addition, while Ebola is not considered an airborne virus, some epidemiologists believe airborne droplets could contribute to the spread in hospital settings.
The 2014 to 2016 outbreak in West Africa has been the largest to date, killing 11,310 and infecting 28,616. The outbreak was contained in Guinea, Sierra Leone, and Liberia, with deaths also occurring in the United States, the United Kingdom, Nigeria and Senegal.
There are currently five known species of the Ebola virus: Zaire Ebola virus, Sudan Ebola virus, Tai Forest Ebola virus, Bundibugyo Ebola virus, and the Reston Ebola virus (which is present in Asia but does not cause human disease). There are two FDA-approved monoclonal antibody treatments for the Zaire Ebola Virus and one vaccine. Vaccines for the other strains are still under research, with a trial in Uganda happening for the Sudan Ebola Virus.
Ebola is one of the Midwest AViDD Center's priority viruses for antiviral development. Project 1 (small molecule entry inhibitors of pandemic viruses) and Project 2 (nanobodies as novel entry inhibitors of pandemic viruses) are dedicated to discovering therapeutics for this disease.
Marburg is thought to be slightly less deadly than Ebola and was first described in the German cities of Marburg and Frankfurt and the Yugoslav capital Belgrade in 1967, where laboratory workers were exposed to infected monkey tissue.
The host animal for Marburg is the Egyptian Fruit Bat, which inhabits a broad swath of Sub-Saharan Africa. A current outbreak in Equatorial Guinea has a case-fatality ratio of up to 88 percent, killing 11 people. The Egyptian Fruit Bats can infect pigs and monkeys, and the Marburg virus spreads like Ebola. A promising vaccine is in a Phase 1 study; however, no approved treatments currently exist.
Lassa Fever

The Lassa fever virus is another virus that can cause severe viral hemorrhagic fever. It is endemic in parts of West Africa, including Sierra Leone, Liberia, Guinea, and Nigeria. Annual infections typically range from 100,000 to 300,000, with deaths amounting to about 5,000. In some areas of Sierra Leone and Liberia, about 10 to 16 percent of people admitted to hospitals annually have Lassa fever, according to the CDC. The mortality rate is 1 percent, but up to 15 percent in severe hospitalized cases. For those that survive, deafness is a common complication. In addition, third-trimester pregnant women and their fetuses are at a heightened risk of death.
As an arenavirus, the virus’s host reservoir is the multimammate mouse, also known as the common African rat. The virus was isolated in 1972, three years after it was first discovered in Nigeria. Since then, four different lineages of the virus have been detected.
The rat can be found in nearly all of West Africa’s natural habitats and readily colonizes homes, frequently breeds with large numbers of offspring, and infests areas where food is stored. The virus is spread through rodent urine and droppings. The infection comes from human contact by touching soiled objects, eating contaminated food, and exposure to open cuts or sores.
It can also be transmitted by inhaling virus particles in air contaminated with infected rodent excretions and kicked up by seemingly innocuous activities like sweeping. Human-to-human transmission may occur after exposure to the virus by an infected individual's blood, tissue, secretions, or excretions, primarily in healthcare settings.
The Lassa fever season is typically observed during the dry season from December to April. The drug ribavirin has been the standard treatment since the 1980s. However, that is due to only one clinical study, and recent research shows that it can be potentially harmful to some patients.
The Midwest AViDD Center has three projects focused on developing antivirals for Lassa fever, which by their nature could easily be stored, stockpiled, and dispersed around endemic hotspots to lower the unacceptably high number of annual deaths. The projects include small molecule and nanobody entry inhibitors and nuclease inhibitors.
Middle East respiratory syndrome coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS)

The 2003 outbreak of SARS was primarily blamed on a zoonotic spillover from palm civets (a cat-like creature most closely related to the mongoose) and bats. It infected 8,000 people from 29 countries, resulting in at least 774 deaths worldwide. SARS is believed to spread in the same airborne fashion as SARS-CoV-2, but it is not as contagious, and infections generally come from close contact.
MERS was caused by an initial spillover from camels, with 27 countries reporting infections since the first outbreak in 2012. Unlike SARS and SARS-CoV-2, the MERS virus resides deep in the respiratory tract, making it less contagious from airborne particles. However, it has a 35 percent case fatality rate.
Neither SARS nor MERS has approved vaccines or treatments. In developing antivirals for SARS-CoV-2, the Midwest AViDD Center is continually analyzing inhibitors with broad-spectrum capabilities that could be used for all coronaviruses.
Nipah Virus

The Nipah virus is bat-borne and most prevalent in fruit bats, also called flying foxes. First discovered in 1998, it can infect humans, pigs, horses, cats, and dogs. Asia sees outbreaks almost every year, with areas and India and Bangladesh typically hit the hardest.
The virus causes encephalitis (swelling of the brain) and has a 40 to 75 percent case fatality rate.
The 1998 outbreak occurred in Malaysia and was concentrated in pig farms. Over one million pigs were culled to prevent more infections. No human-to-human infection was reported during that outbreak.
However, it is a virus of pandemic concern because human transmission is regularly reported in Bangladesh and India, most commonly seen in families, caregivers, or healthcare settings.
Direct contact with infected pigs and bats is the most common mode of infection; however, because fruit bats eat fruits, infections can come from consuming raw date palm sap or tropical fruits contaminated with infected bat saliva or urine.
Currently, no vaccine is available, and monoclonal antibody treatments are in the early stages of development.
Rift Valley fever

In the Eastern Rift Valley in Kenya, where tectonic plates are pulling the earth apart, a mosquito-borne virus can infect humans and livestock such as cattle, sheep, goats, buffalo, and camels.
While direct infection from mosquitos is one way for human infection, blood, body fluids, or tissues of infected animals is also a source of infection. This typically happens during the slaughter or butchering of a diseased animal, during veterinary procedures such as assisting an animal with birth, or by consuming raw or undercooked meat.
Rift Valley fever was first identified in livestock in Kenya in 1910. Since then, it has existed mostly in sub-Saharan Africa, including West Africa and Madagascar. However, the first reported outbreak outside of Africa occurred in 2000 in Saudi Arabia and Yemen.
While the case fatality rate is less than 1 percent, eight to 10 percent of people infected develop severe symptoms, including eye lesions, encephalitis, and hemorrhagic fever. Some researchers believe the fatality rate is up to 30 percent.
Flooding and heavy rainfall are believed to increase the risk of an outbreak. Still, early detection has helped limit the virus' devastation. Climate change could exasperate the spread of the virus, which is expected with mosquito-borne diseases. Human-to-human transmission has not been recorded.
There are no approved treatments for Rift Valley fever.
Zika

Zika is another mosquito-borne virus similar to endemic viruses like dengue fever, yellow fever, Japanese encephalitis, and the West Nile virus. And because of its likeness to these other viruses, it is incredibly hard to detect in rural areas with inadequate healthcare services.
First isolated in Uganda in 1947, it didn't become a global concern until the 2015 to 2016 Zika epidemic in North and South America. Previously believed to be confined to a narrow equatorial belt, the virus somehow crossed the Pacific.
The infection is notoriously associated with a devastating birth defect called microcephaly, which can affect babies born to a woman who becomes infected while pregnant.
Besides infection from mosquitos, Zika can also be transmitted during sex. Only about 20 percent of people infected with Zika will exhibit mild symptoms. Currently, there is no approved treatment or vaccine.
The Midwest AViDD Center has four dedicated research projects for developing antiviral therapeutics for Zika. They include small molecule and nanobody entry, protease, and helicase inhibitors. In addition, because Zika is an RNA virus, there is optimism that broad-spectrum inhibitors can be developed to also protect from dengue fever, yellow fever, Japanese encephalitis, and the West Nile virus.
Disease X

The wildcard. Disease X represents the knowledge that a serious international epidemic or pandemic could be caused by a pathogen currently unknown to cause human disease, according to the WHO. Does that mean it could be the next pandemic or endemic, a new variant, or some otherworldly virus? Nobody knows. But researchers are concerned that the next big one is out there, a spillover away and possibly hidden in some dark cavern.
For example, 219 known viruses can infect humans, according to the NIH. Meanwhile, there are an estimated 3,000,000 to 10 nonillion (10 to the 31st power) viruses on the planet. This includes viruses in seawater, soil, and other non-living entities. Scientists have already identified 887 animal-borne viruses with an associated risk to humans and an estimated minimum of 320,000 viruses in mammals awaiting discovery. Disease X is truly the unknown.